Imagine a tiny, impossibly small world, teeming with activity yet unseen by the naked eye. This is the realm of atoms, the building blocks of everything around us. And within these atoms, electrons dance in intricate patterns, governed by rules we’re only beginning to understand. Today, we’ll embark on a journey into the heart of aluminum, exploring its atomic structure through the captivating lens of the Bohr model.
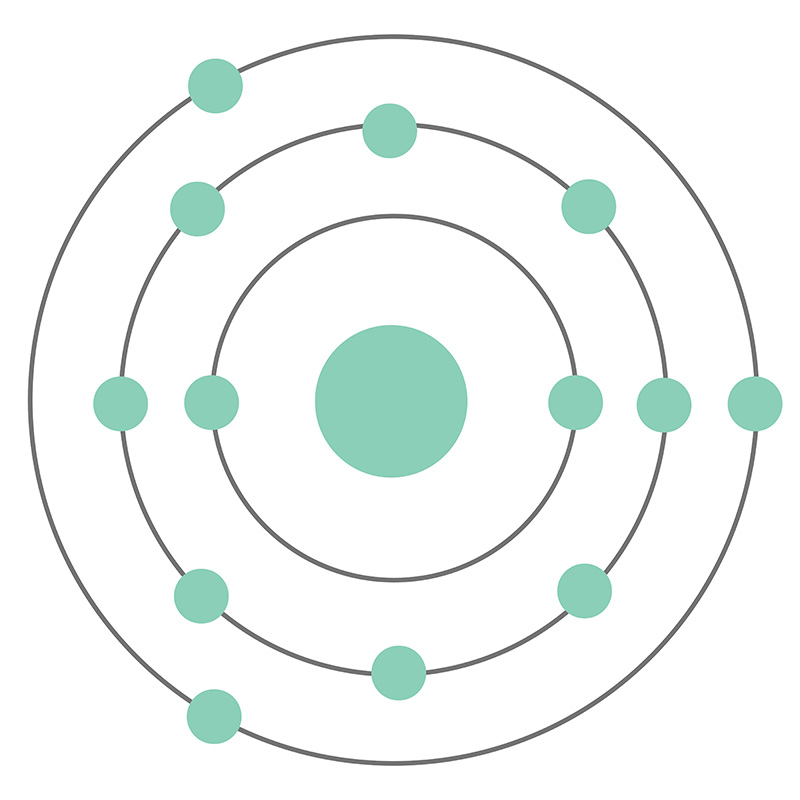
Image:
The Bohr model, though simplified, provides a powerful framework for visualizing the behavior of electrons within atoms. It’s like a miniature solar system, with the nucleus as the sun and electrons orbiting around it in specific, quantized energy levels. This model, while not entirely accurate, serves as a brilliant stepping stone in explaining the fascinating properties of elements like aluminum.
An Atom of Aluminum: A Story in Orbit
Aluminum, a ubiquitous metal found in everything from beverage cans to airplanes, has a fascinating story to tell. Its atomic structure is the key to its versatility and usefulness. Let’s delve deeper into its Bohr model to understand why.
Imagine a tiny nucleus, carrying a positive charge, at the center of the aluminum atom. This nucleus houses 13 protons and 14 neutrons, giving aluminum its atomic number of 13 and an atomic mass of 27. Orbiting this nucleus are 13 electrons, each carrying a negative charge.
But these electrons don’t orbit randomly. They occupy specific energy levels, often likened to “shells” around the nucleus. These shells are named K, L, and M, with the K shell being closest to the nucleus and the M shell farthest away. Each shell can hold a specific number of electrons, with the K shell holding up to 2, the L shell up to 8, and the M shell up to 18.
In the case of aluminum, we have 2 electrons in the K shell, 8 electrons in the L shell, and the remaining 3 electrons in the M shell. This configuration provides valuable insight into the properties of aluminum.
Aluminum’s Brilliance: A Symphony of Electrons
The presence of these 3 electrons in the outermost M shell is what makes aluminum so special. These electrons are loosely bound to the atom and can easily detach, leaving behind a positively charged aluminum ion. This tendency to lose electrons contributes to aluminum’s exceptional properties:
-
Electrical Conductivity: The loosely held electrons in the outer shell act as charge carriers, enabling aluminum to conduct electricity efficiently. This property makes it indispensable in electrical wiring and components.
-
Thermal Conductivity: Aluminum’s atomic structure also facilitates heat transfer. It readily conducts heat, allowing for its use in cooking utensils, heat sinks, and various industrial applications.
-
Lightness and Strength: Its lightweight nature paired with its remarkable strength makes aluminum an ideal material for aircraft construction, automotive parts, and building materials.
-
Corrosion Resistance: Aluminum forms a protective oxide layer on its surface, hindering further corrosion. This makes it suitable for use in outdoor applications and various environments.
Beyond the Bohr: Quantum Mechanics and the Aluminum Atom
While the Bohr model provides a simplified picture, the world of atomic structure is far more intricate. Modern understanding of the atom relies on quantum mechanics, a more sophisticated theory that depicts electrons as probabilistic entities. We now know that electrons are not confined to specific orbits but rather exist in electron clouds, regions of high probability where electrons are likely to be found.
The quantum mechanical model reveals the true complexity of aluminum’s atomic structure. However, the Bohr model remains a valuable tool for visualizing and understanding the fundamental principles of atomic behavior, particularly for introductory learners.

Image:
Exploring Aluminum’s Applications
Aluminum is a truly remarkable element, its versatility extending far beyond everyday objects. Its conductivity, lightness, and corrosion resistance make it a key player in:
-
Aerospace: Its use in aircraft frames and components showcases its ability to withstand extreme conditions while remaining lightweight.
-
Automotive Industry: Aluminum’s lightweight nature and corrosion resistance make it ideal for car bodies, engine components, and various other parts.
-
Packaging: Aluminum’s recyclability and resistance to corrosion make it a preferred material for beverage cans, food packaging, and other consumer products.
-
Construction: Aluminum’s strength and resistance to corrosion make it suitable for building facades, windows, and other structural components.
-
Electronics: Its conductivity enables its use in circuit boards, electrical wiring, and various electronic components.
The Future of Aluminum: Pushing the Boundaries
The world of aluminum continues to evolve with advancements in materials science and engineering. Scientists are exploring new ways to modify its properties, further enhancing its applications.
For instance, research focuses on creating aluminum alloys with enhanced strength and durability, finding new uses in medical implants, high-performance vehicles, and lightweight construction materials.
Aluminum Bohr Model
A Final Thought
As we’ve explored the fascinating story of aluminum through the Bohr model, we’ve witnessed the power of visualizing atomic structure. Whether it’s in the familiar aluminum can we hold in our hand or the sophisticated aircraft soaring through the sky, aluminum’s versatility and resilience are testaments to the power of its atomic structure.
The Bohr model, though simplified, has helped us unlock the key to understanding this remarkable element. It encourages us to appreciate the unseen world of atoms, the intricate dance of electrons, and the remarkable properties that emerge from such a tiny realm.






